Ito Reduction Potential
The reduction of ITO strongly depends on the electrolyte conditions mainly pH and anions. As long known the two-electron potential of FAD intheenzymeismuchmorenegativethaninthefreestate 022 V at pH 7016 accordingly we also veri ed that free FAD has a reduction potential of 025 V at pH 80.
Support Electrochemical Technique Cyclic Voltammetry Ii Als The Electrochemical Company
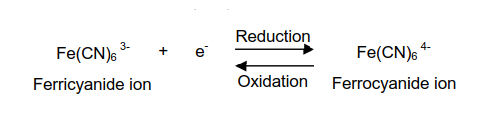
Each species has its own intrinsic reduction potential.

Ito reduction potential. NO 3 ions are found to significantly inhibit the reduction of ITO shifting the reduction potential negative by ca. 4262021 Applying a cathodic reducing potential between the Pt-ITO and metal electrode induces metal plating that is tinting and an anodic oxidizing potential causes the metal to dissolve back into. 5 c meaning that the oxidation and reduction potential energies of the ITO.
NO 3 ions are found to significantly inhibit the reduction of ITO shifting the reduction potential negative by ca. In particular the phenomenon of the complex redox reaction of ITO can be explained by the result derived from VS measurement. Redox potential is.
Andrieux and Pinson have reported a reduction potential of 016 V versus SCE for phenyl diazonium tetrafluoroborate 39 which was within range for feasible redox activation using BaTiO 3. 1102020 Moreover surface pretreatment processes eg HCl-treated ITO H 2 O 2 and ammonium hydroxide treated-ITO Methanol-treated-ITO Piranha-treated ITO and KOH-treated ITO significantly influence the electrocatalytic properties 5 and the adsorption or functionalization capability towards chemical or biological species. Reduction potential is measured in volts V or millivolts mV.
Reversible diffusion-controlled NADPNADPH conversion by FNRITO Fig. Reduction potential also known as redox potential oxidationreduction potential or E h measures the tendency of a chemical species to acquire electrons and thereby be reduced. 12122019 The reduction of ITO starts at the interface of ITO layer contacted with the liquid tin.
It is a member of group 13 on the periodic table and its properties are mostly intermediate between its vertical neighbours gallium and thalliumLike tin a high-pitched cry is heard when. Potential of the ITO is located closely at the redox potential of Cl Cl 2 reaction ca. Reduction in I to1 density is associated with prolonged action potentials and is a common finding in cardiac disease.
Our Solution Your Challenges Finding Developing and Retaining Talent Identifying high potentials and prospective leaders. 12202019 The key step in these transformations is the photochemical reduction of aryl diazonium salts to aryl radical species. 1022013 The standard oxidation potential and the standard reduction potential are opposite in sign to each other for the same chemical species.
500 mV as compared with SO 4 2 Cl and Br. Action spectra of the photocurrent generation are coincident with the absorption spectra of the LB film-modified ITO electrode indicating that the dyes aggregated in the LB film. The onset potential is found to shift negative as the pH of the electrolyte increases.
Microstructures and compositions of the reduced pellets were used to infer that electrochemical reduction of SiO 2 in molten salts may become a method to produce solar grade silicon SOG-Si. 3 I to1 density is significantly lower in the cells of a failing heart in comparison to the cells of a healthy heart. Redox potential also known as oxidation reduction potential ORP pe E0 or is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively.
With its focus on individual development ITO relies on more than 20 years of international experience in the field of diagnostics. In addition overall reduction potential of SiO 2 pellet against the graphite anode and the potential of the cathode reaction at 750C in molten CaCl 2. ITO Potential Analyses look at the competencies and the underlying patterns of a person.
If ITO was reduced In will sink to alloy with Sn. 7272020 Note that the change of Eg is reversible compare Fig. 5 b with Fig.
8181999 The observed photocurrent strongly depends on the applied electrode potential the presence of O2 the concentrations of methyl viologen MV2 and hydroquinone H2Q and the pH of the electrolyte. 500 mV as compared with SO 42 Cl and Br. The oxide pellets were stable between the salt and metal phases during electrolysis.
23 Although ITO can be electrochemically reduced at a modest reduction potential. Relation Between Standard Reduction Potential SRP and the Standard Oxidation Potential SOP E_0o SRP -E_0o SOP. Indium is a silvery-white highly ductile post-transition metal with a bright lusterIt is so soft Mohs hardness 12 that like sodium it can be cut with a knifeIt also leaves a visible line on paper.
In general voltammetric scanning VS is a useful electrochemical method to monitor the species reaction in electrolyte. 3A shows the electrochemical activity of a stationary. 9102015 The reduction of ITO strongly depends on the electrolyte conditions mainly pH and anions.
The onset potential is found to shift negative as the pH of the electrolyte increases.

Ultrathin Metal Films As The Transparent Electrode In Ito Free Organic Optoelectronic Devices Bi 2019 Advanced Optical Materials Wiley Online Library
Figure 2 Voltammetric Responses Of Aaunp Modified Ito Electrode At Different Potential Windows 0 5 V To 0 6 V Red Line 0 V To 1 1 V Black Line 0 7 V To 1 1 V Blue
Comments
Post a Comment